COVID-19 Vaccines and Treatments
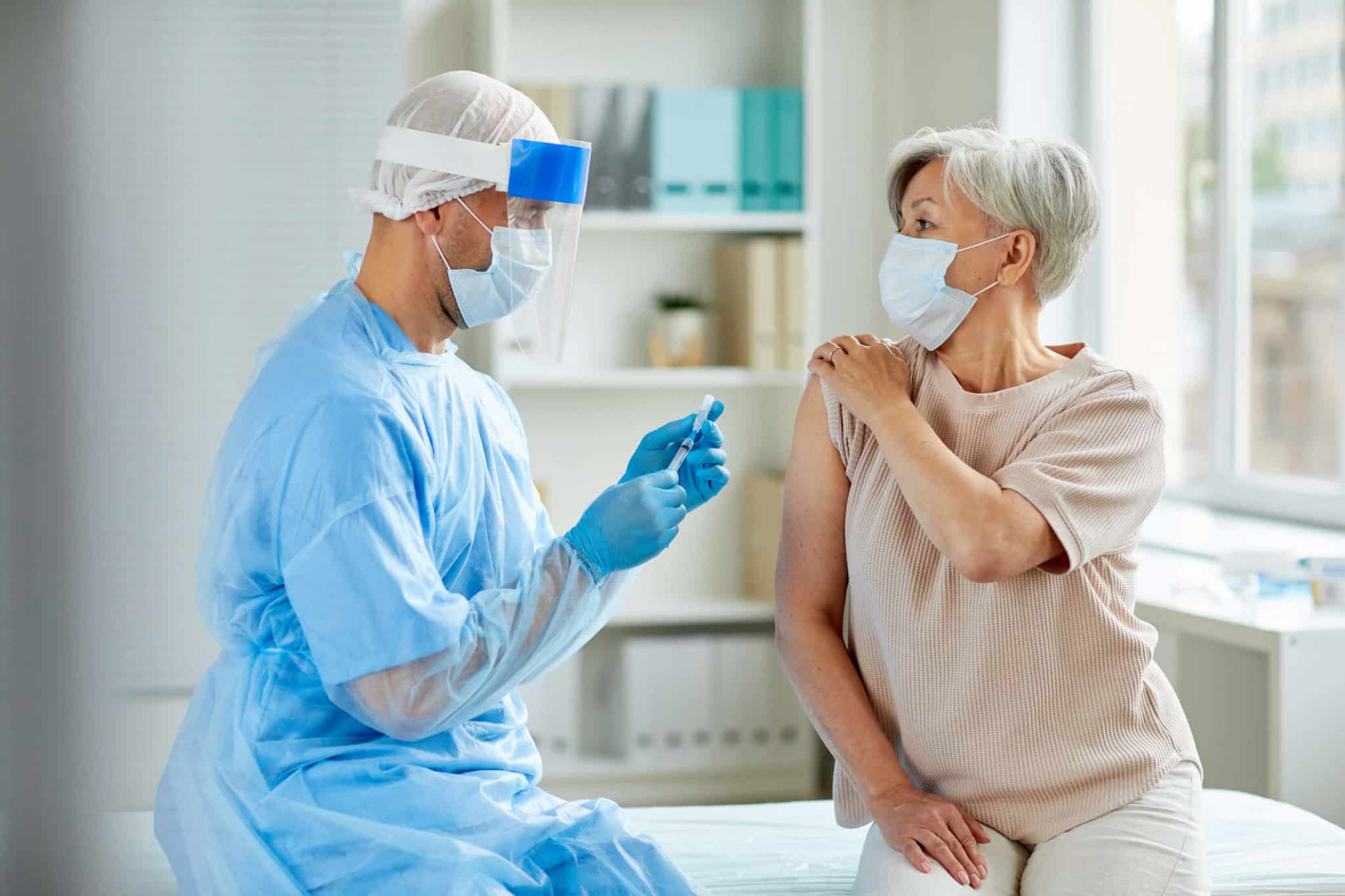
In November 2020, exciting news arrived about COVID-19 vaccines. Pharmaceutical companies like AstraZeneca, Pfizer, and Moderna reported that their vaccines were showing highly effective results. This news was especially good to hear as many parts of the world braced for a strong resurgence in COVID-19 cases.
Here is some insight into the current guidelines set out by the Centers for Disease Control and Prevention (CDC) on what we all should know about vaccination protocols as we move into 2021.
What every person should know about the COVID-19 vaccines
- Safety first – Despite the significant pressure to develop an effective vaccine, no vaccination process will be approved for emergency use unless it is considered as safe as possible. We’re all impatient for the pandemic to end, but the last thing anyone wants is to put people at unnecessary risk.
- Vaccine treatment processes will be flexible – The CDC will work with healthcare associations in each state to develop flexible processes for delivering the vaccine to ensure a steady, efficient rollout. This is about planning for many different possibilities, so be prepared for plans, treatments, and processes to change a bit as new circumstances develop.
- What is Emergency Use Authorization (EUA) – Under emergency situations like the current pandemic, the Food and Drug Administration (FDA) authorizes vaccines for distribution. You can learn more about EUA here.
- Supply of vaccines may be limited – At first, the supply of the vaccine will be limited, and demand will be exceptionally high. Fortunately, the federal government continues to work with vaccine manufacturers to ramp up production and get the necessary logistics in place in order to immunize large groups of people. Remember, this is a vaccination process unlike any other in history, so it won’t be immediately available at every local doctor’s office, clinic or hospital.
- Vaccines will be prioritized – Because it will be impossible to vaccinate everyone at the same time, the most vulnerable groups will get priority . This includes seniors in congregate living settings, frontline workers, hospital personnel, older adults with serious health conditions, and other high-exposure and at-risk groups. Vaccine treatments will become more widely available after these groups receive the shot first.
- The vaccine will be free – Vaccine doses purchased with U.S. taxpayer dollars will be given to the American people at no cost. However, vaccination providers (such as your local pharmacy) will be able to charge an administration fee for giving the shots. Vaccine providers can get this fee reimbursed by the vaccine recipient’s public or private insurance company or, for uninsured associates, by the Health Resources and Services Administration’s Provider Relief Fund.
Health and safety comes first at United Methodist Communities
It’s important to be patient during this time and to focus on the fact that a viable, safe vaccine is on its way. The last year has been frightening and frustrating, but we all have it in us to keep going and to stay focused on what really matters. While we wait for our opportunity to get the vaccine, it is important to continue our protective habits – to wear our masks, wash our hands, social distance with those we love, and most importantly, keep checking on the seniors in our local community.
At Bristol Glen, a senior living community in New Jersey, we understand that this is a concerning time for families with vulnerable loved ones. We’re here to help in any way we can, by providing a safe, supportive environment where the health of our residents and associates come first.
For more information on who we are and how we’re working to keep our seniors safe, contact us today or visit our website at: https://umcommunities.org/unitedforsafety/





